Disc Replacement News- Spinal Kinetics M6
This "Quality of Motion" is a major patient benefit not available in any other implant!
Spinal Kinetics announces completion of 54,000 M6 Artificial Spinal Disc Implants
March 15, 2018 - Internationally, there have been more than 54,000 implants of the M6-C and M6-L since the products were first launched in 2006.
Recently Spinal Kinetics reached completion of its 50,000th M6 Artificial Disc Replacement implant.
Dr. Karsten Ritter-Lang has placed over 6,000 ADR implants and was the first to implant the Spinal Kinetics M6 disc replacement.
July 25, 2017 - http://www.orthospinenews.com/spinal-kinetics-surpasses-50000-implants-of-its-m6-artificial-disc-since-launch/
SUNNYVALE, Calif.–(BUSINESS WIRE)–Spinal Kinetics, Inc., the designer and manufacturer of the innovative M6© artificial disc, today announced total implantations of the company’s M6-C Cervical and M6-L Lumbar discs have now exceeded 50,000 throughout the international markets where the M6 is commercially available. Since the launch of the M6-C in 2006 and the M6-L in 2010, this “natural,” artificial disc design has consistently established itself as a technology of choice among spine surgeons for both cervical and lumbar disc replacement. With this strong surgeon preference, as well as patient demand for the next generation artificial disc technology, the M6 has become an industry leader in the rapidly growing artificial disc market. Additionally, the company is preparing its Pre-Market Approval (PMA) application to the FDA to obtain United States approval to treat single level cervical degenerative disc disease with the M6-C. The M6-C IDE study enrollment has been completed, and the PMA application is scheduled to be submitted to the FDA later this year.
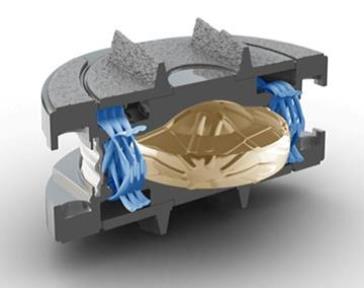
The M6 artificial disc is designed to help patients suffering from degenerative disc disease of the spine; a common cause of chronic neck and back pain. The M6 technology provides an alternative to spinal fusion and is designed to restore natural and physiologic motion to the spine. Preserving motion with the M6 artificial disc provides an opportunity to restore biomechanical function at the treated level after native disc removal, as well as the possibility to reduce subsequent degeneration of adjacent segments. The M6 is the only physiologically designed artificial disc that mimics the anatomic structure of a natural disc by incorporating an artificial visco-elastic nucleus and fiber annulus in its design. Like a natural disc, this unique construct of the M6 allows for shock absorption at the implanted level, as well as provides a controlled range of motion when the spine transitions in its combined complex movements. Extensive biomechanical studies at world renowned institutions have continuously confirmed the M6’s ability to replicate natural physiologic motion.
“We are extremely proud as an organization to reach the 50,000 implant level and are appreciative of the spine surgeon community in adopting the M6 technology,” states Tom Afzal, President and CEO of Spinal Kinetics. “We believe this milestone continues to validate the market demand for more advanced artificial disc technologies that are designed to closely mimic a natural disc’s motion characteristics, ultimately benefiting patient outcomes. Disc replacement in the cervical and lumbar spine is not solely about how much motion an artificial disc provides, but more importantly, the quality of that motion and how that motion can affect other surrounding structures like facets and adjacent segments. The M6’s ability to replicate a natural disc’s quality of motion and biomechanics is what continues to differentiate it against all other artificial discs in the market.”
About Spinal Kinetics, Inc.
Founded in 2003, Spinal Kinetics is a privately held medical device company focused on partnering with spine surgeons to develop innovative and practical motion preservation systems for treating degenerative diseases of the spine. The M6-C cervical and M6-L lumbar artificial discs have rapidly established themselves among the leading artificial discs available due to the unique biomechanical properties that replicate the motion of a natural disc and the positive clinical outcomes for patients. The company is located in Sunnyvale, California.
The M6 is the only artificial disc that replicates the anatomic structure and biomechanics of a natural disc by incorporating both an artificial nucleus and annulus. In the US, Spinal Kinetics has successfully completed an FDA IDE Pilot Study of the M6-C in patients with both single and two level disease, and has received approval from the FDA to initiate an IDE Pivotal Study. "Motion preservation with the M6 disc is a very exciting advance for spine surgery technology," states Carl Lauryssen, MD, Chief of Spine Surgery at Olympia Medical Center in Beverly Hills. "We have seen extremely high success rates with our patients from the FDA Pilot Study, and believe the technology is increasing the quality of life for patients with degenerative disc disease beyond what was previously available to them".
Unlike early Disc Replacement designs, the Spinal Kinetics M6 artificial disc is designed to replicate the structure and performance of a natural disc. Its innovative design incorporates an artificial nucleus to allow shock absorption and a woven fiber annulus for graded variable motion resistance in all directions.
These characteristics accurately replicate the natural disc, allowing the implant to work in concert with the remaining human discs. Unlike earlier "ball-in-socket" implants, with the M6 disc replacement the resulting natural functionality of the entire spinal curve will provide the best chance for a full recovery. In addition, future complications will be eliminated by reducing adjacent level degeneration and strain on the muscles and ligaments.



