Disc Replacement in the US
THE BACKBONE
OF YOUR BUSINESS
ARE YOUR BOOKS
This "Quality of Motion" is a major patient benefit not available in any other implant!
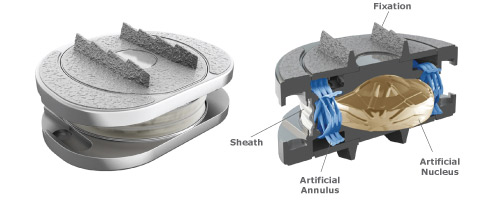
The Spinal Kinetics M6 Lumbar artificial disc replacement offers an innovative option compared to other artificial lumbar disc replacement because of its unique design, which is based on the qualities of the natural disc.
The M6 Lumbar Disc Replacement is the only artificial disc that incorporates an artificial nucleus (made from polycarbonate urethane) and a woven fiber annulus (made from polyethylene). The M6-L artificial nucleus and annulus are designed to provide the same physiologic motion characteristics of a natural disc. Extensive biomechanical testing with the M6-L artificial lumbar disc has demonstrated equivalent Quality of Motion compared to the healthy disc.
Together, the M6 Lumbar’s artificial nucleus and annulus provide compressive capabilities and a controlled range of natural motion in all 6 degrees of freedom. This “natural” motion is designed to provide the freedom to move your back naturally.
The M6 Lumbar Disc Replacement has two titanium outer plates with keels for anchoring the disc into the bone of the vertebral body. These outer plates are coated with a titanium plasma spray that promotes bone growth into the metal plates, providing long-term fixation and stability of the disc in the bone.
Many people ask why was I not offered disc replacement in the US?
You can get disc replacement in the US, but there are several limitations.
The first set of limitations come from the FDA. Since the studies were typically only done on patients with one bad disc the FDA has for the most part limited use of disc replacement in the USA to one disc surgeries.
The second limitation is age, again the FDA only allowed patients under 60 in the trials so the approval only allows for disc replacement surgery for those under 60 years of age.
If these FDA limitations were not enough to eliminate most patients from being offered disc replacement in the US as it turns out many US insurance carriers still consider disc replacement experimental and specifically exclude the disc replacement surgery from payment.
So let’s say you fit within these guidelines and are in fact offered disc replacement, now you must consider what type of product you are being offered!
Most disc replacement products approved by the FDA and offered in the US are what we consider outdated. These early free floating ball in socket type disc replacement implants have several draw backs. Surgical complications like implant migrations and implant subsidence were fairly common in early designs. Long term complications like facet joint wear and post op pain are also common due to the unnatural and uncontrolled freedom of motion and fixed center of rotation in these early disc replacement designs.
This means that patients who may be candidates for disc replacement from a medical point of view are often not offered disc replacement in the US.
These patients are offered fusion surgery or are treated with less effective micro discectomy and laminectomy, sometimes called laser spine surgery, or older implant designs.
Newer advanced implant options resolved these issues and now provide shock absorption, better stability, motion control and improved end plate fixation.
The most advanced of these new disc replacement devices is the Spinal Kinetics M6 Disc Replacement.
Now available for the lumbar and cervical spine and for multi-level disc replacement surgeries



